BDGP Resources
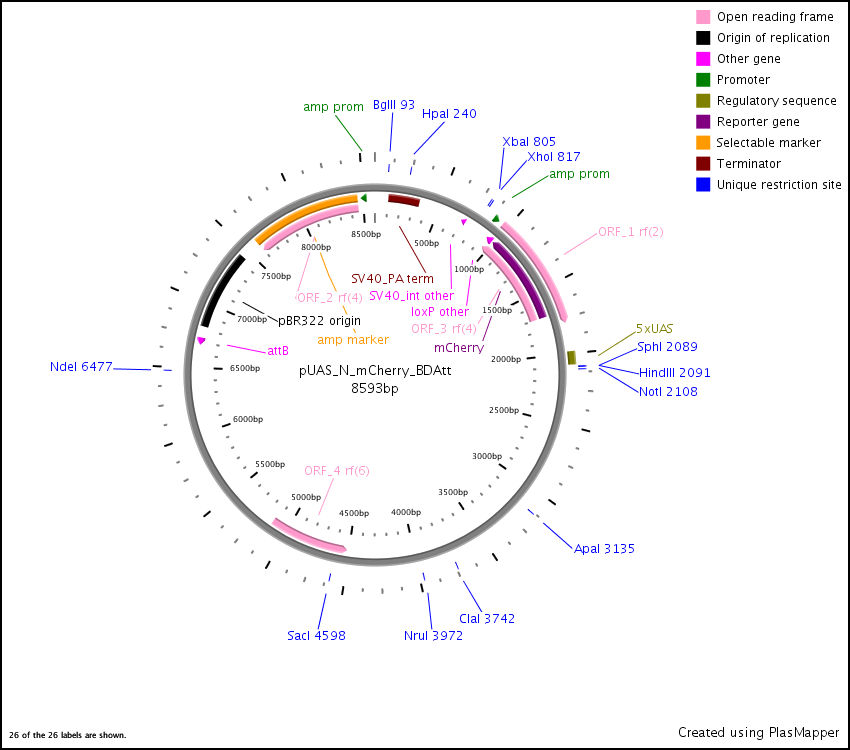
pUAS-N-mCherry-BD-attB Vector
pUAS-N-mCherry-BD-attB.fasta
>pUAS-N-mCherry-BD-attB vector ORGANISM pUAS-N-mCherry-BD-attB vector)
by Charles Yu Summary: Generating the expression vectors, pUAS-C-EGFP-BD-attB, pUAS-C-mCherry-BD-attB, pUAS-N-EGFP-BD-attB and pUAS-N-mCherry-BD-attB took three successive steps. The first step is to BD-adapt the pUAST vector (Brand and Perrimon, 1993) with a C-terminus or N-terminus BD cassette. The second step is to insert new EGFP or mCherry fluorescent tags into the BD-adapted pUAST vector. The third step is to transfer a portion of the tagged, BD-adapted pUAST vector into pBDP vector (Pfeiffer et al., 2008), a modular minimal cloning vector for specific in vivo genomic targeting of Drosophila melanogaster using PhiC31 integrase.
Method: To generate the C-terminus tagged vectors, the pUAST expression vector (Brand and Perrimon, 1993) was cut with NotI and XbaI . The pUAST-C-TAP-BD expression vector (Stapleton) was cut with NotI and XbaI to release a 256bp fragment containing loxP, chloramphenicol promoter and splice acceptor sequences. This 256bp C-terminus BD cassette was inserted into the linearized pUAST vector by overnight ligation. The resulting C-terminus BD-adapted pUAST vector was linearized again by cutting it with XbaI. To generate the N-terminus tagged vectors, the pUAST expression vector was cut with EcoRI and XhoI. The pUAST-N-TAP-BD expression vector (Stapleton) was cut with EcoRI and XhoI to release a 167bp fragment containing loxP and chloramphenicol promoter sequences. This 167bp C-terminus BD cassette was inserted into the linearized pUAST vector by overnight ligation. The resulting N-terminus BD-adapted pUAST vector was linearized again by cutting it with EcoRI. The CDS of the EGFP and mCherry fluorescent tags were obtained by PCR from the pEGFP-C1 (Clontech) and pRSET-B-mCherry (Invitrogen) vectors, respectively. C-terminus fluorescent tags were amplified using XbaI-tailed PCR primers, while N-terminus tags were amplified using EcoRI-tailed PCR primers (see below). The resulting PCR products were agrose gel-purified and cut with either XbaI (C-terminus tag) or EcoRI (N-terminus tag). Following ethanol precipitation, the XbaI digested C-terminus fluorescent tags were inserted into the XbaI linearized BD-adapted pUAST vector by overnight ligation. The EcoRI digested N-terminus tags were inserted into the EcoRI linearized BD-adapted pUAST vector by overnight ligation. The resulting C- or N-terminus fluorescent-tagged and BD-adapted pUAST vectors are cut with BamHI to release the portion of the vector containing the 5x UAS promoter, hsp70, BD cassette, fluorescent tag and SV40 terminator sequences. Agarose gel-purify the BamHI digested fragments. We linearized the pBDP vector by cutting it with BamHI and ethanol precipitated the digested product. We inserted the purified C- or N-terminus fluorescent-tagged and BD-adapted BamHI fragments from the pUAST vectors into the linearized pBDP vector by overnight ligation. The resulting vectors are pUAS-C-EGFP-BD-attB, pUAS-C-mCherry-BD-attB, pUAS-N-EGFP-BD-attB and pUAS-N-mCherry-BD-attB.
Sequences: 256bp C-terminus BD cassette: 5'-GGCCGCATAACTTCGTATAGCATACATTATACGAAGTTATAGATCCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTGGATCTCGAGCTCAAGCTTCGAATTCAGGGTTTCCTTGACAATATCATACTTATCCTGTCCCTTTTTTTTCCACAGCTACCGGTCGCGT-3' 167bp N-terminus BD cassette: 5'-AATTCATAACTTCGTATAGCATACATTATACGAAGTTATAGATCCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTC-3' XbaI-tailed PCR primers: C-mCherry-FWD 5'-cccctctagaGTGAGCAAGGGCGAGGAGGATAACATG-3' C-mCherry-REV 5'-ggggtctagaTTACTTGTACAGCTCGTCCATGCCGC-3' C-EGFP-FWD 5'-cccctctagaGTGAGCAAGGGCGAGGAGCTGTTC-3' C-EGFP-REV 5'-ggggtctagaTTACTTGTACAGCTCGTCCATGCCGAG-3' EcoRI-tailed PCR primers: mCherry-N-FWD 5'-ccccgaattcacaccATGGTGAGCAAGGGCGAGGAGGAT-3' mCherry-N-REV 5'-ggggaattcccCTTGTACAGCTCGTCCATGCCGCC-3' EGFP-N-FWD 5'-ccccgaattcacaccATGGTGAGCAAGGGCGAGGAGCTG-3' EGFP-N-REV 5'-ggggaattcccCTTGTACAGCTCGTCCATGCCGAG-3'
